The history of the existence of Sodium bicarbonate (Baking soda) in human life is much more than we can give a year to it, it actually exists from the first date of each born child.
The acidity of Blood, toxicity of water resources, kidney disease, fungal infection, influenza, hypertension, and even Cancers, in this modern world, are what make you need Sodium Bicarbonate as a vital chemical product to stay alive.
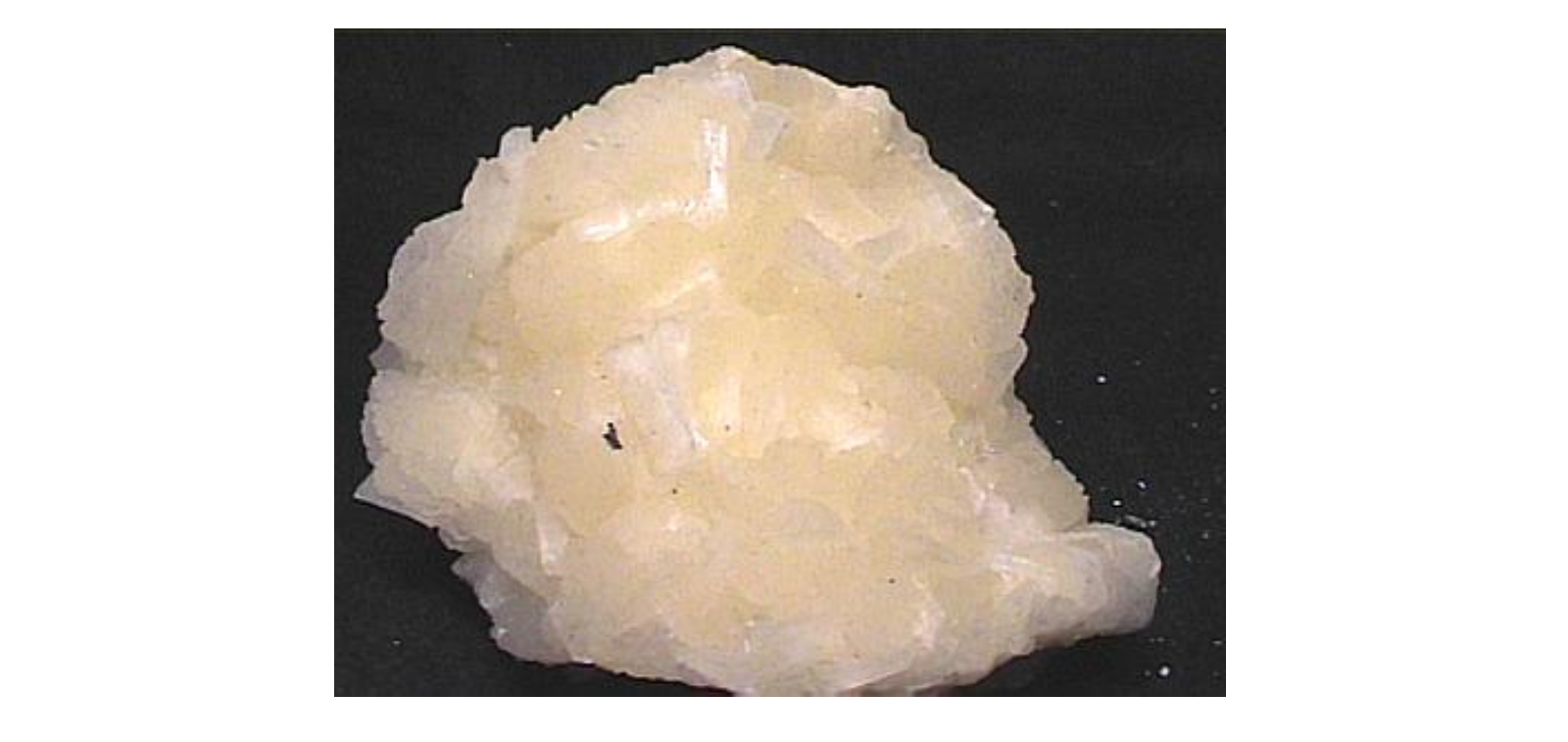
Before the 1860s and developing Solvay process or ammonia-soda process by Belgian chemist “Ernest Solvay” most of the sodium bicarbonate we know in the form of these days is a natural mineral form in nahcolite y Trona deposits. Nahcolite or bicarbonato is a soft, colorless or white carbonate mineral with the composition of bicarbonato de sodio which planar chains of carbonate groups linked by hydrogen bonds.
Nahcolite or bicarbonato commonly forms by the reaction of carbon dioxide with the mineral trona in evaporated lake basins. Apparently, there are two ways to mine for trona and Nahcolite deposits, one is a ‘room-and-pillar method that involves carving out underground rooms supported by pillars. The mineral is scraped off the walls and removed by a conveyor belt. The other is a liquid-injection method, where miners inject hot water underground to dissolve the minerals, pump out the liquid, and then evaporate the water to get at the leftover crystals.
 A process for making bicarbonato de sodio from Nahcolite-rich solutions utilizes solubility suppression with the addition of salt. Hot water pumped into a Nahcolite-rich formation, which may contain salt (NaCl) and other impurities in excess of 6% by weight, dissolves the Nahcolite (NaHCO3) and salt. The dissolved Nahcolite is brought to the surface as a pregnant brine liquor containing Nahcolite, salt, and other impurities, which is then de-gassed under pressure. The pregnant liquor is then mixed with a salt slurry and its temperature reduced to saturate it with salt and force precipitation of Nahcolite out of the pregnant liquor. Carbon dioxide (CO2) is injected while mixing the pregnant liquor with the salt to facilitate precipitation and conversion of any sodium carbonate (Na2 CO3) present into sodium bicarbonate (NaHCO3). The bicarbonato de sodio (NaHCO3) is dewatered and filtered to form a sodium bicarbonate (NaHCO3) filter cake, which is washed with fresh cold water to remove salt (NaCl), sodium carbonate (Na2 CO3), and other impurities as a salt-rich brine filtrate, and the filter cake is dried to produce a high grade of natural sodium bicarbonate (NaHCO3). The filtrate may be processed to produce hot water condensate which can then be used to dissolve the Nahcolite, and the salt slurry produced can be used to saturate the pregnant liquor and force Nahcolite precipitation, and a portion may be dried and used as a saleable by-product.
A process for making bicarbonato de sodio from Nahcolite-rich solutions utilizes solubility suppression with the addition of salt. Hot water pumped into a Nahcolite-rich formation, which may contain salt (NaCl) and other impurities in excess of 6% by weight, dissolves the Nahcolite (NaHCO3) and salt. The dissolved Nahcolite is brought to the surface as a pregnant brine liquor containing Nahcolite, salt, and other impurities, which is then de-gassed under pressure. The pregnant liquor is then mixed with a salt slurry and its temperature reduced to saturate it with salt and force precipitation of Nahcolite out of the pregnant liquor. Carbon dioxide (CO2) is injected while mixing the pregnant liquor with the salt to facilitate precipitation and conversion of any sodium carbonate (Na2 CO3) present into sodium bicarbonate (NaHCO3). The bicarbonato de sodio (NaHCO3) is dewatered and filtered to form a sodium bicarbonate (NaHCO3) filter cake, which is washed with fresh cold water to remove salt (NaCl), sodium carbonate (Na2 CO3), and other impurities as a salt-rich brine filtrate, and the filter cake is dried to produce a high grade of natural sodium bicarbonate (NaHCO3). The filtrate may be processed to produce hot water condensate which can then be used to dissolve the Nahcolite, and the salt slurry produced can be used to saturate the pregnant liquor and force Nahcolite precipitation, and a portion may be dried and used as a saleable by-product.
As obvious about mining and its costs, Solvay process in producing synthetic bicarbonato de sodio solves the problems and make it so much easier to have such a vital product. In the Solvay process, Sodium carbonate is prepared by passing carbon dioxide through ammonia that gives ammonium carbonate. Subsequently, ammonium carbonate is converted to ammonium hydrogen carbonate that reacts with sodium chloride to precipitate out sodium hydrogen carbonate. Sodium hydrogen carbonate later forms sodium carbonate.
